![]()
LOCATION! LOCATION! LOCATION!
Press Release, 12-40
U.S. Commerce Department to Open Four Regional U.S. Patent Offices
For the First Time, USPTO Will Expand Operations Outside Washington, DC, to:
Detroit!
Dallas!
Denver!
AND
Silicon Valley!
WASHINGTON – Acting U.S. Commerce Secretary Rebecca Blank and Under Secretary of Commerce for Intellectual Property and Director of the U.S. Patent and Trademark Office (USPTO) David Kappos today announced plans to open regional USPTO offices in or around Dallas, Texas, Denver, Colorado, and Silicon Valley, California. These offices are in addition to the already-announced first USPTO satellite office to open on July 13 in Detroit, Michigan. The four offices will function as hubs of innovation and creativity, helping protect and foster American innovation in the global marketplace, helping businesses cut through red tape, and creating new economic opportunities in each of the local communities. Next week, Acting Secretary Blank and Under Secretary Kappos will travel to each of the newly selected cities to meet with local businesses, entrepreneurs and public officials to discuss the new office openings.
“Intellectual property protection and innovation are engines of economic growth and the bedrock of America’s private sector,” said Acting U.S. Commerce Secretary Rebecca Blank. “The Obama administration is committed to making certain our businesses and entrepreneurs have the resources they need to grow, create jobs and compete globally. These new offices are an historic step toward further advancing our world’s best IP system, and reinforcing the United States as the number one destination for innovation capital, and research and development around the world.”
The offices announced today will help the USPTO attract talented IP experts throughout the country who will work closely with entrepreneurs to process patent applications, reduce the backlog of unexamined patents, and speed up the overall process, allowing businesses to move their innovation to market more quickly, and giving them more room to create new jobs.
“By expanding our operation outside of the Washington metropolitan area for the first time in our agency’s 200-plus year history, we are taking unprecedented steps to recruit a diverse range of talented technical experts, creating new opportunities across the American workforce,” said David Kappos, Director of the USPTO. “These efforts, in conjunction with our ongoing implementation of the America Invents Act, are improving the effectiveness of our IP system, and breathing new life into the innovation ecosystem.”
Patents are a significant factor in private sector job creation. In fact, the U.S. Commerce Department issued a recent report finding that IP-intensive industries are the source – directly or indirectly – of 40 million jobs, contributing $5.06 trillion to the U.S. economy in 2010.
Selection of the four sites was based upon a comprehensive analysis of criteria including geographical diversity, regional economic impact, ability to recruit and retain employees, and the ability to engage the intellectual property community. The Leahy-Smith America Invents Act of 2011 (AIA), signed into law by President Obama in September, requires the USPTO to establish regional satellite locations as part of a larger effort to modernize the U.S. patent system over the next three years.
The USPTO is working to develop concept of operations for the three newly-announced locations based on the Elijah J. McCoy Detroit Office and will develop best practices based on this model over the coming months and years. The Detroit office will employ approximately 120 individuals in its first year of operations. The USPTO also seeks to identify and maximize the unique regional strengths of all four offices to further reduce the backlog of patent applications and appeals.
The USPTO team plans to begin site procurement activity and establish a timeline for the three newly-announced locations in the coming months.
For additional background on the selection criteria and methodology, please click here.
For non-press inquiries pertaining to the satellite site selection, please contact Azam Khan, USPTO deputy chief of staff at azam.khan@uspto.gov.
“FIRST TO FILE” AMERICA INVENTS ACT LEGISLATIVE HISTORY GUIDE

Stop at Starbucks, get your favorite venti coffee and several treats(you’ll need them), find your favorite chair, and grab your Ipad, and link to Social Science Research Network. Joe Matel, who, currently, is the Judiciary Committee Counsel to Senator Jon Kyl, has presented his two part article, titled, “A Guide to the Legislative History of the America Invents Act (AIA)” to examine the origins and the legislative commentary on the provisions of the Leahy-Smith America Invents Act, a comprehensive patent-reform law that the POTUS signed and enacted on September 16, 2011. The AIA adopts the first-to-file system of patent priority, modifies the defintion of prior art, and creates several new post-issuance administrative proceedings and amends existing proceedings. Part I of the article focuses on the AIA’s changes to sections 102 and 103 of title 35 and related issues. The article is organized by the sections of title 35 that are significantly amended by the AIA, and then by the uncodified sections of the AIA.
Citation: (Matal, Joe, A Guide to the Legislative History of the America Invents Act: Part I of II (2012). 21 Fed. Cir. B.J. 435 (2012). Available at SSRN: http://ssrn.com/abstract=2064740)
Thank you to Patently O for bringing this to my attention.
MICRO ENTITY INVENTORS MAY RECEIVE 75% DISCOUNT ON PATENT FEES

USPTO Issues Proposed Rules to Implement the Micro Entity Provision for Paying Discounted Patent Fees
The USPTO has published proposed rules related to micro entity provisions in the Leahy-Smith America Invents Act. Smaller innovators qualifying for micro entity status receive a 75 percent discount on patent fees. The micro entity proposed rules were published in the Federal Register on May 30, starting a sixty-day public comment period. Read more at USPTO.
Micro Entity Status Inventors
Patent application filers may be able to qualify for Micro Entity status and qualify for 75% discount of patent filing fees. The Leahy-Smith America Invents Act, (AIA) signed by President Obama on September 16, 2011, creates this new status that entitles micro entity patent filers to a remarkable 75% discount on fees. Currently the regular fee for filing a provisional patent application is $250, the Small Entity fee is $125 and the Micro Entity fee would be $62.50. Although, this status is supposed to be available as of the date of enactment of the AIA, the USPTO is not expected to place the Micro Entity fee system into effect until 2013.
To qualify as a Micro Entity, the filer must be a Small Entity and must meet the following criteria:
(1) The applicant has not been named as the inventor on a total of more than four utility patents (regular utility patents, not provisional patent applications), design patents or plant patents. This also does not include certain international PCT applications and applications owned by a previous employer. In addition, the applicant had to have had a gross income in the previous year of less than three times the median household income reported by the Bureau of the Census (The median household income has been hovering around $50,000 for the past two years.) In the event that the patent application has been assigned, the assignee had to have a gross (not net) income of less than three times the U.S. median household income; or
(2) the majority of the patent filer’s employment income is from an Institution of Higher Learning, or the applicant has assigned, or is obliged to assign the patent to an Institution of Higher Learning. An Institution of Higher Learning is a public or non-profit accredited institution that admits post-secondary students for programs of not less than 2 years.
The United States Patent and Trademark Office e – Office Action Debut
Join the Successful e-Office Action Program Now!
e-Office Action is an e-mail communication option that allows you to receive Office communications faster than by traditional postal mail and maintain an online electronic record of notifications, all through the convenience of Private PAIR.
Benefits of e-Office Action
• Faster notification than by postal mail
• Less risk of delayed or lost Office communications
• Ability to view and download correspondence in Private PAIR that will expedite the availability of docketed Office communication.
• Reduced paper management in your office
Enrollment in e-Office Action is easy and takes only a few minutes from the Private PAIR Customer Number details tab.
For more information about the e-Office Action Program and how to take advantage of its benefits visit the e-Office Action web site or call the Patent Electronic Business Center (EBC) at 1-866-217-9197 (toll-free).
Adam “MCA” Yauch of the Beastie Boys Sadly Loses Life to Cancer

Adam “MCA” Yauch, the rapper, musician, activist, filmmaker and film distributor who founded pioneering hip-hop trio the Beastie Boys, as well as indie film distrib company Oscilloscope Laboratories, died on Friday in New York after a three-year battle with cancer. He was 47. Born in Brooklyn in 1964, Yauch was an only child of an architect and a homemaker. He began playing bass guitar and formed his first band at age 17, eventually creating hardcore punk act the Beastie Boys. After some early changes, the group’s primary lineup consisted of Yauch, Michael “Mike D” Diamond, Kate Schellenbach and Adam Berry. The group played the New York punk circuit and released EP “Polly Wog Stew” in 1982.
After tours opening for Madonna and Run DMC, the group released the Rubin-produced, Def Jam-released “Licensed to Ill” in November of 1986, quickly spawning singles “(You Gotta) Fight for Your Right (To Party),” “No Sleep Till Brooklyn,” “Paul Revere” and “Brass Monkey.” The album would remain at the pinnacle of the Billboard album chart for five straight weeks.
(Please see full story http://www.variety.com/article/VR1118053529)
Young V.A. Hospital Researcher Dies From Probable Bacterial Meningitis Contamination
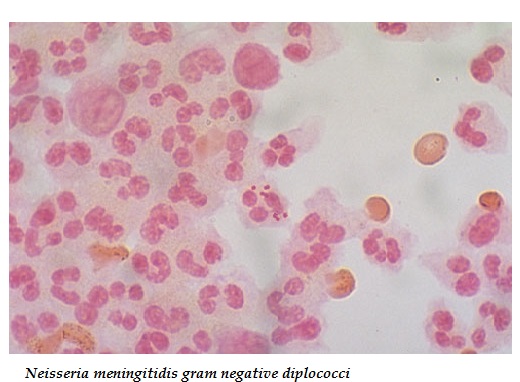
The death of a young researcher at the San Francisco Veteran Affairs Medical Center has died from probable contamination with Neisseria meningitis Group B strain. Richard Din, 25, died Saturday, just 17 hours after developing acute symptoms of meningitis. He had complained of flu-like symptoms Friday night that worsened quickly into a full-body rash, as reported by Doug Sovern for CBS San Francisco.
For more information regarding bacterial meningitis, please see CDC; WHO.
SUPREMES HOLD FRCPs OUTPLAYS SECTION 145
SUPREME COURT OF THE UNITED STATES DECIDES KAPPOS v. HYATT
[April 18, 2012]
JUSTICE THOMAS delivered the opinion of the Court.
Under the Patent Act of 1952, if a Patent and Trade Office (PTO) examiner denies a patent application, 35 U. S. C. §131, the applicant may file an administrative appeal with the PTO’s Board of Patent Appeals and Interferences, §134. If the Board also denies the application, the applicant may appeal directly to the Court of Appeals for the Federal Circuit under §141. Alternatively, the applicant may file a civil action against the PTO Director under , which permits the applicant to present evidence that was not presented to the PTO.
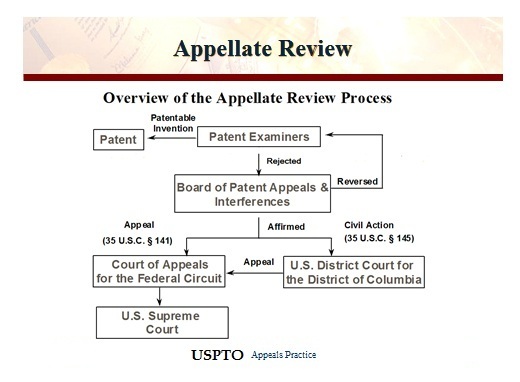
Respondent Hyatt filed a patent application covering multiple claims. The patent examiner denied all of the claims for lack of an adequate written description. Hyatt appealed to the Board, which approved some claims but denied others. Pursuant to §145, Hyatt filed a civil action against the Director, but the District Court declined to consider Hyatt’s newly proffered written declaration in support of the adequacy of his description, thus limiting its review to the administrative record. Applying the deferential “substantial evidence” standard of the Administrative Procedure Act (APA) to thePTO’s factual findings, the court granted summary judgment to the Director. On appeal, the Federal Circuit vacated the judgment, holding that patent applicants can introduce new evidence in §145 proceedings, subject only to the limitations in the Federal Rules of Evidence and the Federal Rules of Civil Procedure. It also reaffirmed its precedent that when new, conflicting evidence is introduced, the district court must make de novo findings to take such evidence into account.
Raw Scraped Ground Tuna Product Identified as Source of Salmonella bareilly Multistate Outbreak
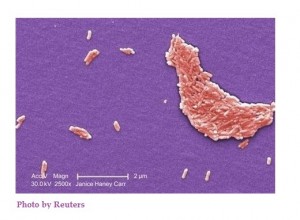 Salmonella bareilly gram negative rod source of raw scraped ground yellowfin tuna.
Salmonella bareilly gram negative rod source of raw scraped ground yellowfin tuna.
FDA reports:
Moon Marine USA Corporation voluntarily recalls frozen raw yellowfin tuna product
“Nakaochi Scrape” associated with a multistate outbreak of Salmonella Bareilly infections
- April 17, 2012 UPDATE:Moon Marine USA Corporation (also known as MMI) of Cupertino, Calif., is voluntarily recalling all frozen raw yellowfin tuna product from India, labeled as Nakaochi Scrape AA or AAA. Nakaochi Scrape is tuna backmeat, which is specifically scraped off from the bones, and looks like a ground product.
- The product is not available for sale to individual consumers, but may have been used to make sushi, sashimi, ceviche and similar dishes available in restaurants and grocery stores.
- The company name and Nakaochi Scrape AA or AAA were printed on boxes of the product when it was initially sold to distributors. However, the boxes may have been broken into smaller lots for further sale and may not be available to the end retailer or consumer. Therefore, the tuna may not be readily identifiable by retail outlets or by consumers as being from the implicated lots.
- The Nakaochi Scrape AA and AAA from MMI was sold through distributors to restaurants and grocery stores that make sushi, and has been linked to the ongoing outbreak of SalmonellaBareilly, which has caused 116 illnesses in 20 states and the District of Columbia to date.
- Many of the people who became ill reported eating raw tuna in sushi as “spicy tuna.”
- If you purchase “spicy tuna” or other sushi, sashimi, ceviche, or similar dishes that might contain Nakaochi Scrape from a restaurant or grocery store, check with the establishment to make sure that it does not contain raw recalled product from Moon Marine USA Corporation, also known as MMI. When in doubt, don’t eat it.
- Consumers who think they might have become ill from eating possibly contaminated raw Nakaochi Scrape should consult their health care providers.
Center for Disease Control (CDC) reports:
- A total of 116 persons infected with the outbreak strain of SalmonellaBareilly have been reported from 20 states and the District of Columbia.
- The number of ill persons identified in each state is as follows: Alabama (2), Arkansas (1), Connecticut (5), District of Columbia (2), Florida (1), Georgia (5), Illinois (10), Louisiana (2), Maryland (11), Massachusetts (8), Mississippi (1), Missouri (2), New Jersey (7), New York (24), North Carolina (2), Pennsylvania (5), Rhode Island (5), South Carolina (3), Texas (3), Virginia (5), and Wisconsin (12).
- 12 ill persons have been hospitalized, and no deaths have been reported.
- Collaborative investigation efforts of state, local, and federal public health agencies indicate that a frozen raw yellowfin tuna product, known as Nakaochi Scrape, from Moon Marine USA Corporation is the likely source of this outbreak of Salmonella Bareilly infections. Nakaochi Scrape is tuna backmeat that is scraped from the bones of tuna and may be used in sushi, sashimi, ceviche, and similar dishes. The product looks like raw ground tuna.
- Consumers should not eat the recalled product, and retailers should not serve the recalled raw Nakaochi Scrape tuna product from Moon Marine USA Corporation.
- This investigation is ongoing. CDC and state and local public health partners are continuing surveillance to identify new cases.
To date, a total of 7 clusters at restaurants or grocery stores have been identified where 2 or more unrelated ill persons reported eating in the week before illness. In each cluster, at least one ill person reported eating sushi purchased at the restaurant or grocery store. These clusters are located in 5 states: Connecticut, Maryland, Rhode Island, Texas, and Wisconsin.
Several methods were used to evaluate the association between tuna and illness in this outbreak. To estimate the frequency of consumption of tuna and “spicy tuna” among all sushi eaters, investigators assembled a comparison group from 1) diners who ate at one of the cluster restaurants or grocery stores or 2) a restaurant where a single ill person, who was judged to have a reliable memory, recalled consuming sushi only once in the week before illness. Records were collected on sushi orders that were placed at the same time of day (lunch or dinner) and as close to the date when the ill person ate at the restaurant.
This study is ongoing. Thus far, information has been collected from 4 clusters at restaurants or grocery stores. The proportion of sushi orders that contained tuna as an ingredient averaged 61% (ranging from 43% to 71%). The proportion of sushi orders that contained “spicy tuna” as an ingredient averaged 37% (ranging from 29% to 53%). These data suggest there is an association between illness and consumption of sushi made with tuna, and specifically “spicy tuna.”
Public health and regulatory officials also are visiting restaurants and grocery stores associated with ill persons and collecting information about the ingredients used in “spicy tuna” recipes. Based on available information from 5 of the 7 restaurant or grocery store clusters about ingredients used to make “spicy tuna”, raw tuna was found to be a common ingredient among all 5 clusters.
State and local public health and regulatory officials are working with the U.S. Food and Drug Administration (FDA) to conduct a traceback of tuna. FDA has selected 4 of the clusters, which are located in Connecticut, Rhode Island, Texas, and Wisconsin, as the focus of the initial investigation. Information to date indicates that all 4 received the same imported frozen raw Nakaochi Scrape tuna product from a single tuna processing facility in India.
This investigation is ongoing. CDC and state and local public health partners are continuing surveillance to identify new cases. Further investigation is ongoing to identify possible sources of contamination and whether any other tuna products are linked with illness. CDC will update the public on the progress of this investigation as information becomes available.
It’s a product found in raw fish dishes such as sushi or sashimi, called “raw yellow-fin tuna scrape,” produced by one California fish processor.
Moon Marine USA Inc. voluntarily recalled more than 58 thousand pounds of scrape, which is raw meat literally scraped from the spine of tuna.
The FDA urged consumers to avoid sushi unless they are sure that it does not come from the affected processor.
“When in doubt, dont eat it,” said FDA spokesperson Curtis Allen.
Contact Elizabeth Reilly, Attorney
If you or a family member became ill with a Salmonella infection after consuming contaminated sushi please call your physician or go to your nearest hospital emergency room. Thereafter, if you are interested in pursuing a legal claim, contact Elizabeth Reilly for a free confidential claim evaluation. As a former Medical Technologist, I have isolated many varieties of Salmonella species, other food borne or water borne bacteria, and parasites which cause injury. Kindly, contact me at:
253-330-6420 or patentpendinglaw@gmail.com
Sushi, Sashimi, or Similar Foods Suspected in Salmonella bareilly Outbreak In 19 States

 Center for Disease Control (CDC)
Center for Disease Control (CDC)
Salmonella bareilly was isolated for the first time in 1928 (Bridges & Scott, 1931). Although identified in India for many years (Ganguli, 1958) its importance as a human pathogen causing both gastroenterities (Raichowdhuri et al. 1983; Agarwal et al. 1983) and nosocomial infections (Panhorta & Agarwal, 1982) has only been recently recognized.
A total of 100 individuals infected with the outbreak strain of Salmonella bareilly have been reported from 19 states and the District of Columbia. The 7 new cases are from Connecticut (1), Illinois (1), Maryland (2), New Jersey (1), Pennsylvania (1), and Wisconsin (1).
Among 100 persons for whom information is available, illness onset dates range from January 28 to March 25, 2012. Ill persons range in age from 4 to 78 years, with a median age of 31. Forty-seven percent of patients are female. Among 51 persons with available information, 10 (20%) reported being hospitalized. No deaths have been reported.
Illnesses that occurred after March 8, 2012, might not be reported yet due to the time it takes between when a person becomes ill and when the illness is reported. Salmonellosis is an infection with bacteria called Salmonella. Most persons infected with Salmonella develop diarrhea, fever, and abdominal cramps 12 to 72 hours after infection. The illness usually lasts 4 to 7 days, and most persons recover without treatment. However, in some persons, the diarrhea may be so severe that the patient needs to be hospitalized.
Initial Announcement
CDC is collaborating with public health officials in many states and the U.S. Food and Drug Administration (FDA) to investigate a multistate outbreak of Salmonella serotype bareilly infections. Salmonella Bareilly is an unusual serotype of Salmonella. Public health investigators used DNA “fingerprints” of Salmonella bacteria obtained through diagnostic testing with pulsed-field gel electrophoresis, or PFGE, to identify cases of illness that may be part of this outbreak. They used data from Pulse Net, the national subtyping network made up of state and local public health laboratories and federal food regulatory laboratories that performs molecular surveillance of foodborne infections.
A total of 93 individuals infected with the outbreak strain of Salmonella bareilly have been reported from 19 states and the District of Columbia. The number of ill people identified in each state with the outbreak strain is as follows: Alabama (2), Arkansas (1), Connecticut (4), District of Columbia (2), Georgia (4), Illinois (8), Louisiana (2), Maryland (8), Massachusetts (4), Mississippi (1), Missouri (1), New Jersey (6), New York (23), North Carolina (2), Pennsylvania (2), Rhode Island (4), South Carolina (3), Texas (3), Virginia (5), and Wisconsin (8).
Among 93 persons for whom information is available, illness onset dates range from January 28 to March 23, 2012. Ill persons range in age from 4 to 78 years, with a median age of 31. Forty-six percent of patients are female. Among 51 persons with available information, 10 (20%) reported being hospitalized. No deaths have been reported.
Illnesses that occurred after March 4, 2012, might not be reported yet due to the time it takes between when a person becomes ill and when the illness is reported. This takes an average of 2 to 3 weeks.
Investigation of the Outbreak
State public health officials are interviewing ill persons to obtain information regarding foods they might have eaten and other exposures in the week prior to illness. On initial interviews, many of the ill persons reported consuming sushi, sashimi, or similar foods in a variety of locations in the week before becoming ill. Among 51 ill persons for whom information is available, 35 (69%) reported consuming sushi, sashimi, or similar foods in the week before illness onset. This percentage is higher than expected compared with results from a survey of healthy persons in which 5% of persons reported consuming sushi, sashimi, or ceviche made with raw fish or shellfish in the 7 days before they were interviewed. The investigation into specific types of sushi is ongoing.
The investigation has not conclusively identified a food source. Investigation is ongoing into individual food items and their sources. CDC, FDA, and state and local public health partners are continuing surveillance to identify and interview other ill persons about the foods they ate. CDC will update the public on the progress of this investigation as information becomes available.
AP Video: Center for Food Safety

